diagram orbital|orbital diagram example : Cebu Orbital diagrams are pictorial representations of the electron configuration, showing the . Free cancellations on selected hotels. 21 Cheap Hotels in Lapu-Lapu. Browse through 21 Lapu-Lapu hotel reviews and hotel photos with ease.
PH0 · understanding orbital diagrams
PH1 · orbital diagram worksheet with answers
PH2 · orbital diagram for all elements
PH3 · orbital diagram example
PH4 · orbital diagram calculator
PH5 · how to ground state orbital diagram
PH6 · how to find orbital diagram
PH7 · how to draw orbital diagrams
PH8 · Iba pa
“Floods have washed away entire villages, wiping out homes, farmlands, and the critical infrastructure necessary to support a swift recovery and movement of people, goods, and much-needed .
diagram orbital*******Orbital diagrams are diagrams that show the energy and spin of electrons in atoms or .
A p orbital along the y axis is labeled p y and one along the z axis is a p .diagram orbital orbital diagram exampleBy convention, elements are organized in the periodic table, a structure that captures .
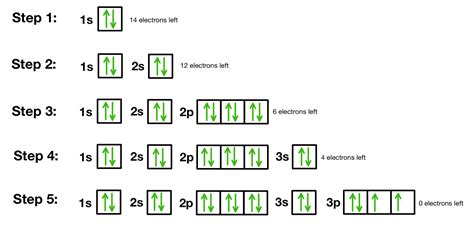
Orbital Diagrams. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. This is done by .Orbital diagrams are pictorial representations of the electron configuration, showing the .An orbital is a space where a specific pair of electrons can be found. We classified the .
An orbital is the quantum mechanical refinement of Bohr’s orbit. In contrast to his .Each orbital in an atom is characterized by a set of values of the three quantum numbers n, ℓ, and mℓ, which respectively correspond to the electron's energy, its orbital angular momentum, and its orbital .This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n.An orbital is a space where a specific pair of electrons can be found. We classified the different Orbital into shells and sub shells to distinguish them more easily. . And so in this diagram or this a visualization right over here, I've depicted the one shell and then I've also depicted the two shells. So this is a shell right over here .Orbital yang sama akan memiliki bilangan kuantum n, l, dan m yang sama. Yang membedakannya hanya bilangan kuantum spin (s).” Sumber: kompas.com. Hal ini berarti bahwa setiap orbital maksimum berisi .
The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals. This means that the second shell can hold 8 total electrons. Every orbital is a shape (that can be determined by a trigonometric function I .Cara Membuat Diagram Orbital. Orbital adalah bagian dari subkulit atom, sebagai daerah yang paling mungkin ditempati elektron. Dalam penyusunan diagram orbital, sebuah elektron disimbolkan dengan anak panah menghadap ke atas atau menghadap ke bawah. Anak panah yang menghadap ke atas biasanya melambangkan elektron dengan spin . The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms.
The 2s orbital would be filled before the 2p orbital because orbitals that are lower in energy are filled first. The 2s orbital is lower in energy than the 2p orbital. There are 5 d orbitals in the d subshell. A p orbital can hold 6 electrons. Based off of the given information, n=4 and ℓ=3. Thus, there are 3 angular nodes present.The result is a diagram that looks like the one drawn below in Figure 5.2.1.2 5.2.1. 2. The bond order is calculated using the molecular orbitals (we can ignore atomic orbitals). There are 10 electrons in binding orbitals and 6 electrons in antibonding orbitals). This gives a bond order of 12(10 − 6) = 2 1 2 ( 10 − 6) = 2.
In quantum mechanics, an atomic orbital ( / ˈɔːrbɪtəl /) is a function describing the location and wave-like behavior of an electron in an atom. [1] This function describes the electron's charge distribution around the atom's nucleus, and can be used to calculate the probability of finding an electron in a specific region around the nucleus.An atomic orbital, which is distinct from an orbit, is a general region in an atom within which an electron is most probable to reside. The quantum mechanical model specifies the probability of finding an electron in the three-dimensional space around the nucleus and is based on solutions of the Schrödinger equation.
The Basics of Orbital Diagrams. There are different types of orbitals, that all have different energy levels. These orbitals are filled with electrons (the amount of electrons depends on which element you are .
The 1s, 2s and 2p z orbitals of oxygen are symmetric (i.e., unchanged) with respect to all three symmetry operations. They are given the symmetry classification a 1.The 2p x orbital, since it possesses a .
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau.Orbital Shape Diagram: 1: 0: s: 2: 1: p: 3: 2: d: 4: 3: f: How Do Electrons Occupy the Orbital Space? S Orbital. At the first main energy level, when n = 1, the only sublevel, or orbital, possible is the s-orbital, which has a sphere shape. P Orbital. When n = 2, two sublevels are possible: these are the s-orbital and p-orbitals.
Konfigurasi Elektron, Diagram Orbital, Contoh Soal, dan Pembahasannya. Gurubagi.com. Atom terdiri atas inti dan elektron yang beredar mengitarinya menurut lintasannya masing-masing. Untuk mengetahui bagaimana lintasan elektron tersebut, maka dapat kita melihat penyebaran elektron dalam kulit-kulit elektron melalui konfigurasi .
diagram orbital Konfigurasi Elektron, Diagram Orbital, Contoh Soal, dan Pembahasannya. Gurubagi.com. Atom terdiri atas inti dan elektron yang beredar mengitarinya menurut lintasannya masing-masing. Untuk mengetahui bagaimana lintasan elektron tersebut, maka dapat kita melihat penyebaran elektron dalam kulit-kulit elektron melalui konfigurasi .An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its principal energy level and .

At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. A p orbital is rather like 2 identical balloons tied together at the nucleus. The diagram on the right is a cross-section through that 3-dimensional region of space.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .
Quais as novidades do Quizur? Como criar um quiz de personalidade? Como criar um quiz de certo e errado? Como criar uma lista? O que é uma Categoria? O que é uma TAG? O que é o autocorretor? Como excluir um conteúdo? Como excluir minha conta? Já tenho login no Quizur. Preciso me cadastrar novamente? Não estou conseguindo logar com a .
diagram orbital|orbital diagram example